Project and Risk Management
● Strong project management team with over 10 years of project management experience
● Experienced team with profound knowledge of oligonucleotide API manufacturing and pharmaceutical science, and high-quality and efficient communication skills
● Management Process and a Project Information Confidentiality Management System
● Emphasize the importance of risk control and cycle control
● Establish a regular Technical Exchange Program and Project Update/Report System
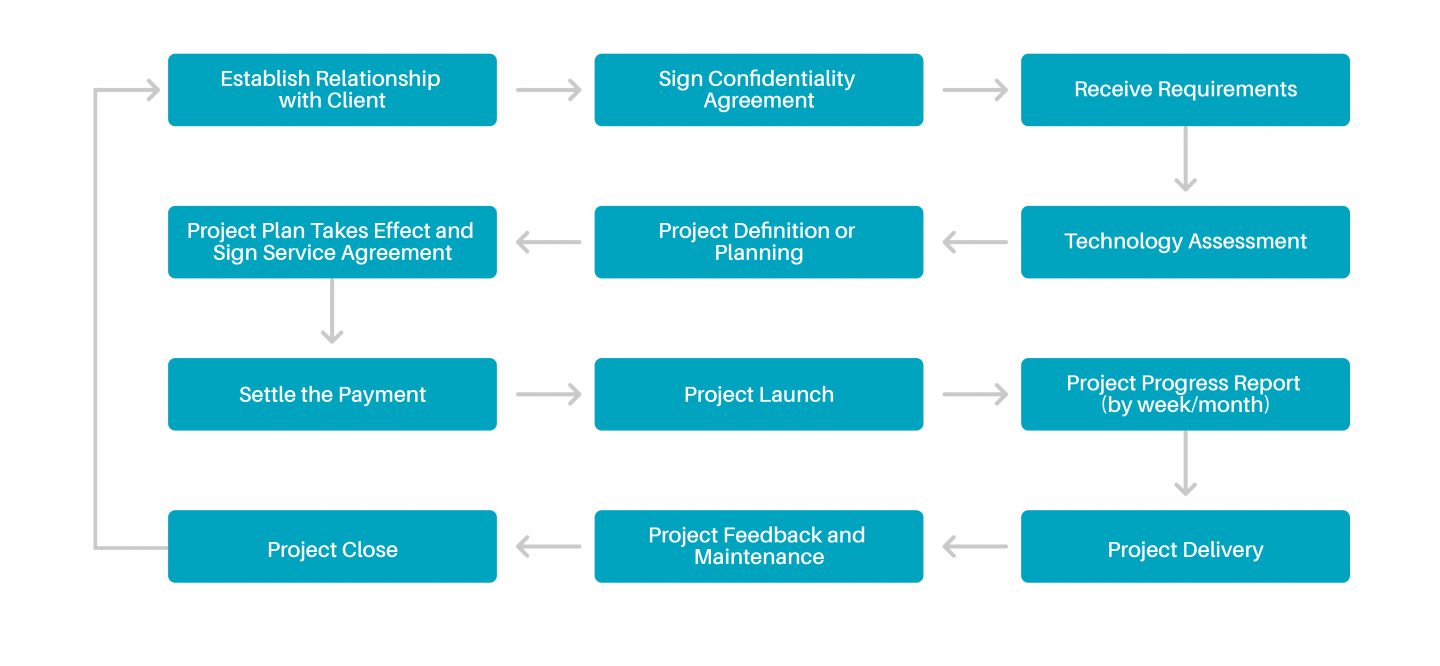
Project Management Process for API Production